What Best Describes an Ionic Bond
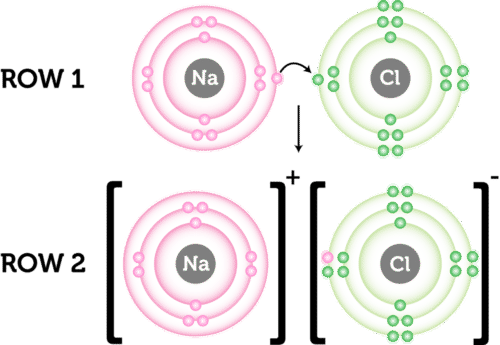
One atom gains an electron while the other atom loses an electron and an electrostatic force attracts them. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds.
The transfer of electrons results in attractive forces between molecules.
. Always a whole number Which situation best describes an ionic bond. Biology 21062019 2200 misscheoneyo. Between which kinds of atoms is an ionic bond formed.
The atom that donate the electron become a positively charged ion while the atom that received the atom become a negatively charged ion. If an ionic bond is stronger than a dipole-dipole interaction how can water dissolve an ionic compound. In conclusion the statement which best describes an ionic bond is that an ionic bond involves a metal that transfers one or more electrons to a nonmetal.
This bond is the result of the nonmetal desiring electrons to fill out its valence and a metal. The sharing of electrons forms strong bonds between ions. Ionic Bond Vs Covalent Bond.
Which of the following best describes what happens when an ionic bond forms. What best describes how an ionic bond forms. The transfer of electrons forms strong bonds between ions.
B The ionic bond is weakened by the ion-dipole interactions and ionic repulsion ejects the ions from the crystal. This bond is the result of the nonmetal desiring electrons to. A The ion-dipole interactions of a bunch of water molecules gang up on the strong ionic bond and pull it into the solution.
An ionic bond is a type of bond that forms between a metal and nonmetal. When a metal forms bond with a non metal the bond is an ionic bond. Which statement best describes how an ionic bond forms.
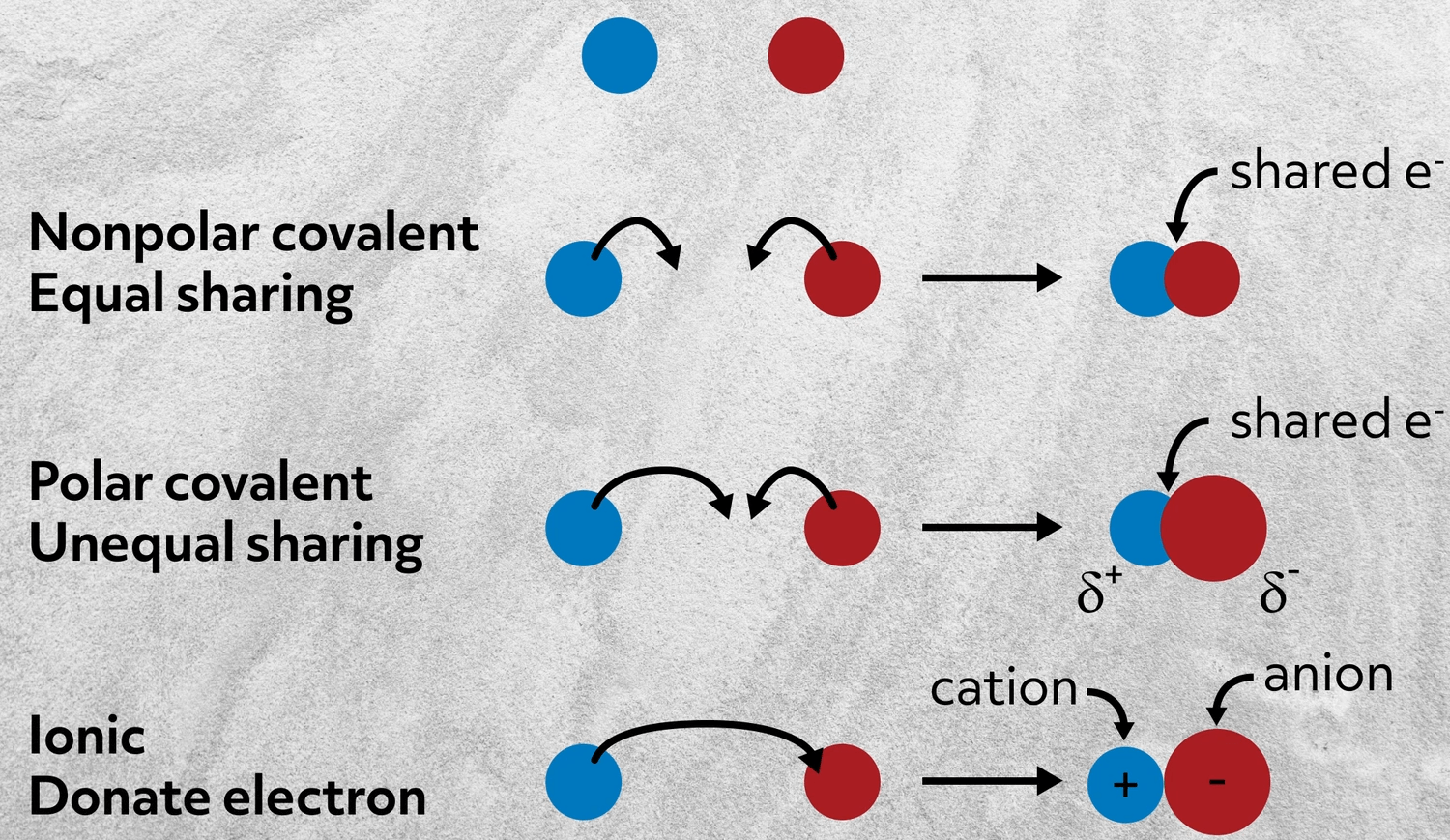
Which statement best describes the relationship between an allele and a gene. An allele is a variation of a gene that can be expressed as a phenotype. The covalent bond is a bond formed when two atoms share one or more electron pairs.
An ionic bond is a type of chemical bond that generally involves a metallic element transferring one or more electrons to a non-metallic element. The bond between the carbons in the molecule H₂CCH₂ is stronger than the bond between the carbons in HCCH. 1 Get Other questions on the subject.
Ionic bonding is the complete transfer of valence electrons between atoms and is a type of chemical bond that generates two oppositely charged ionsSimilarly nonmetals that have close to 8 electrons in its valence shell tend to readily accept electrons to achieve its noble gas. Which best describes how an ionic bond forms. A metal and nonmetal react to form an ionic bond.
An allele is the part of a gene. A metal and nonmetal react to form an ionic bond. Two atoms one atom which is more electronegative than the other exchange electrons and the charges hold the atoms together.
Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. What best describe how an ionic bond form. The sharing of electrons results in attractive forces between molecules.
The correct option is A. Click to see full answer Similarly you may ask what best describes an ionic bond. Each atom contributes an equal number of electrons towards the bond formation.
The question sayswhich statement best describes how an ionic bond forms. The bond dissociation. Lowest ratio of atom in an ionic compound.

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Comments
Post a Comment